Quality Assurance
-
Quality Assurance
Keeping customers satisfaction first, we contribute to the daily medical practices using the production control system in compliance with the Pharmaceutical and Medical Device Act, consistent from sales, design, production thorough quality assurance.
As for the general medical devices (Class1), documentation of the medical package inserts and notification of the sales and manufacturing to the government are done on our own. 
-
Various Devices for Quality Control
Our Osaka office is newly equipped with digital measuring instruments, an autoclave sterilization apparatus, a UV color laser marking device and packaging equipment for building a quality assurance system in compliance with Quality Management System.
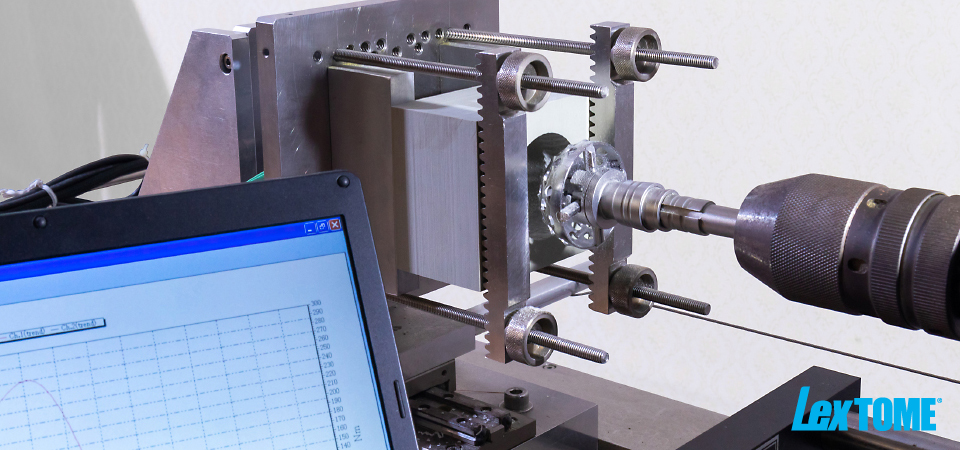
[Cutting Performance Test Device] Meister,Inc./HOS-1
[2-Component Sensor Fz, Mz] Kistler Holding AG/type9345B
-
[3D Scanner CMM]
KEYENCE CORPORATION/VL-800
-
[Rockwell Hardness Testing Machine]
Mitutoyo Corporation/HR-430MR
-
[Image Dimension Measurement System]
KEYENCE CORPORATION/IM-8030T specification:360˚ multi-surface measurement with a rotary index head
-
[Image Dimension Measurement System]
KEYENCE CORPORATION/IM-7030
-
[Digital Microscope]
HIROX CO., LTD./HRX-01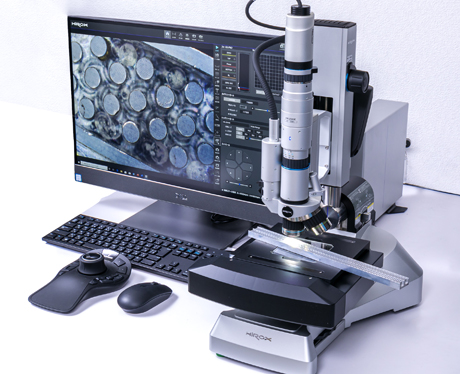
-
[Digital Microscope]
HIROX CO., LTD./RH-2000
-
[Ultrasonic Hardness Tester]
JFE Advantech Co., Ltd./SH-22-S004a
-
[Ultrasonic Hardness Tester]
JFE Advantech Co., Ltd./SH-22-J1a
-
[Digital Torque Gauge]
IMADA CO.,Ltd./HTGA-2N
-
[Handheld XRF Analyzer]
Olympus Corporation/VCR-CCC-A3-J-JA〉
-
[Driver Torque Meter]
IMADA. CO.,LTD./I-80
-
[Force Tester]
A&D Company,Limited/MCT-2150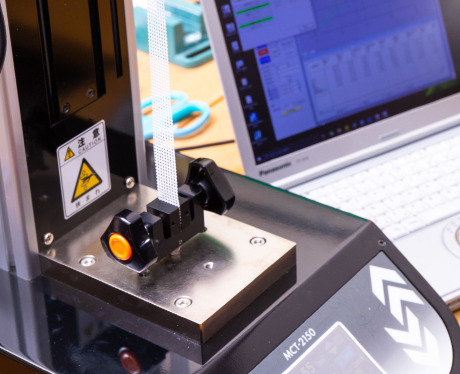
-
[3D Scanner CMM]
KEYENCE CORPORATION/VL-570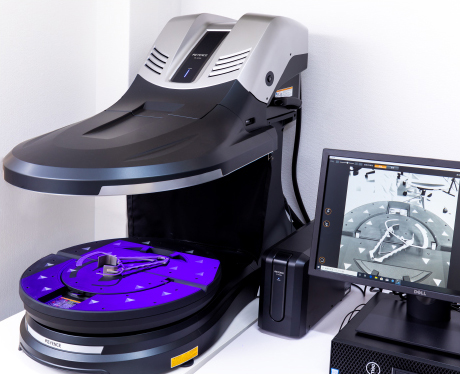
-
[Autoclave]
HIRAYAMA Manufacturing Corp./HG-50LB
